Evaluation of the effectiveness and safety of the BodyDetox24
An open-label pilot test
Background
BodyDetox24 is a product composed and designed to purify the body by detoxification. It is composed of vitamins and selected herbs, whose active ingredients are extracted by a special extraction procedure, a patent-pending proprietary technology of Hamida Pharma Inc, California, USA. Contrary to other detoxifying products which are said to act within weeks, BodyDetox24 acts in a very short time, i.e. within 24 hours. Although the action might seem drastic, the product has been tested in toxicological tests and documented to cause no harm to healthy people.
Study design
Healthy volunteers were invited to participate in this pilot test by advertisements in a local newspaper and the test was performed at and supervised by a local out patient clinic in Sandefjord, Norway. The test aimed at investigating changes (if any) in blood chemistry parameters and the personal experience of participants by notations in a form for self-evaluation.
| Inclusion criteria: | Healthy, not seriously ill volunteers |
| Exclusion criteria: | Pregnancy or lactating, gall stone problems, taking vital medicines. (Taking vital medicines was defined as using medicines which had to be taken at regular intervals and where the intake could not be delayed for 4 hours). |
Due to the action of BodyDetox24 it is suitable to use the product in non-working days, i.e. during week-ends or holidays. Before entering the test (a couple of days before a week-end, generally on a Wednesday, Thursday or Friday) participants had blood samples drawn for a standard clinical laboratory analysis at day 0. At the same time they were given a self-evaluation form to be filled in before and after using BodyDetox24. After having fulfilled a 24 hour course of BodyDetox24 during the week-end participants returned to the local clinic for a second blood sample within 3 days, i.e. no more than a week was to pass from start to finish of the test.
The self-evaluation form consisted of 4 parameters, which participants should estimate on a 5-step scale before and after taking BodyDetox24. The scale was numbered from 0 (No) to 4 (Very much) for the parameters Energy, Sleep, and General well-being. For the parameter Tiredness the 5-step scale was reversed.
Results
1. Clinical laboratory analysis
Forty one healthy volunteers, 18 males and 23 females, entered and completed the test. An additional group of 15 healthy volunteers participated in the self-evaluation section of the study, so that results from this part of the study are based on a total of 56 included participants.
From the standard clinical laboratory analyses the following parameters were judged to be relevant in relation to BodyDetox24:
| Parameter | Indfication of | Laboratory reference value |
|---|---|---|
| gamma-glutamyl transferase, (GGT) Alanine Aminotransferase (ALAT) |
Liver status |
75 U/L |
| Creatinine | Kidney status | 45 – 90 mmol/L |
| Potassium (K+) Sodium (Na+) Magnesium (Mg+2) |
Electrolyte status | 3,6 – 5,0 mmol/L 137 – 145 mmol/L 0,71 – 0,94 mmol/L |
| Lactate dehydrogenase (LDH) | Safety | 205 U/L |
Gamma-Glutamyl Transferase, GGT
An increase in GGT is seen in liver damage, often (but not exclusively) in connection with use of alcohol or drugs. High levels of GGT in the blood may be a sign of liver disease or damage to the bile ducts. Bile ducts are tubes that carry bile in and out of the liver. Bile is a fluid made by the liver. It is important for digestion.
| Parameter | Mean value before treatment | Mean value after treatment | Norma |
|---|---|---|---|
| The whole group | 55 U/L | 45 U/L | 75 U/L |
|
Participants with values above |
377 U/L | 301 U/L | 75 U/L |
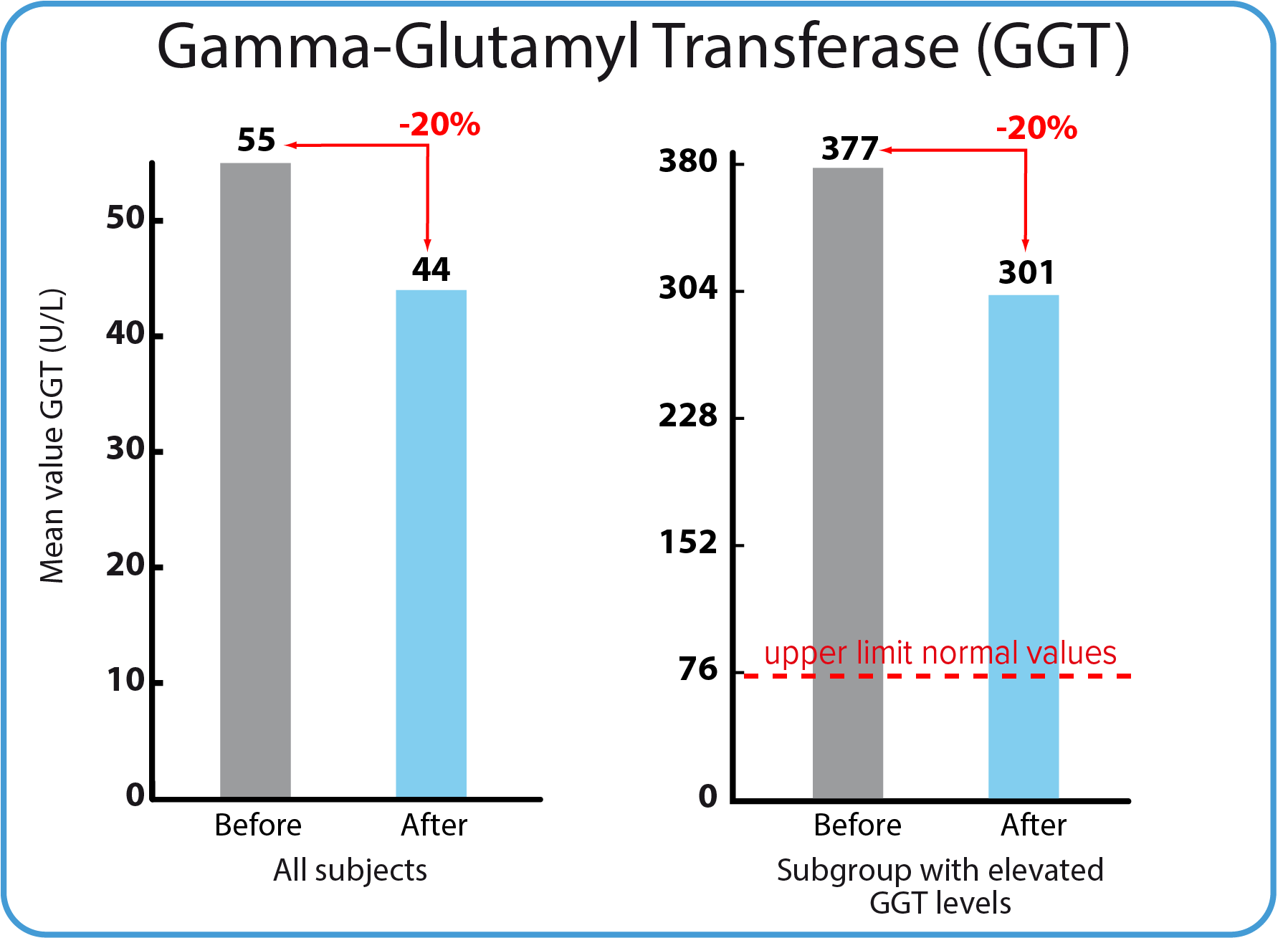
| Mean values for the whole study group were below the reference and reduced by 20 % | A small group of participants had mean values above the reference. They were reduced by 20 % but not below the reference. |
Alanine Aminotransferase, ALAT
s-ALAT is a marker of liver function. Increased values are seen at liver damage, e.g. at alcohol consumption. No particular change in mean values was recorded in the healthy study group, who all were below reference value.
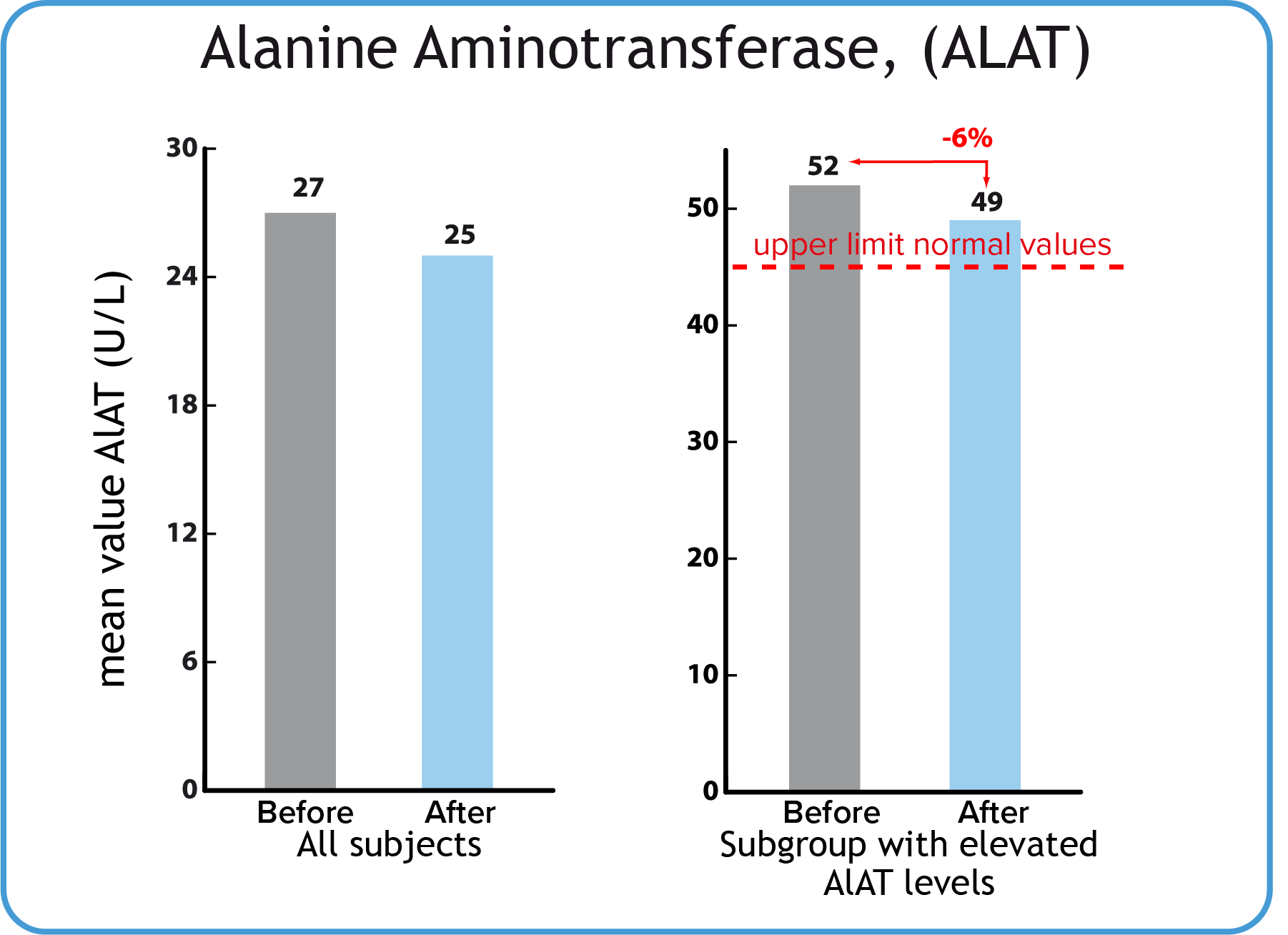
| Mean ALAT values for the whole group was below the reference value (< 45 U/L) before as well as after using BodyDetox24. | A small number of participants had initial ALAT values above the reference value. Mean values for this group were reduced by 6 %. (Right graph). |
Creatinine
Creatinine, which is a product of metabolism, is excreted from the body through the kidneys in the urine, being, apart from urea, one of the main nitrogen compounds. It is formed in the body as a result of non-enzymatic breakdown of creatine phosphate. The amount of creatinine excreted per day depends on the muscle mass and is characteristic of a given organism. On average, about 14-26 mg of creatinine per kilogram of body weight is excreted in the urine daily. Creatinine in serum is increased in disturbed kidney function. An increase in s-creatinine for an individual indicates a decreasing kidney function and – inversely – a reduced s-creatinine value for an individual indicates a better kidney function.
| Subjects | Before treatment | After treatment | Norm |
|---|---|---|---|
| The whole group | 119 U/L | 95 U/L | 45 - 90 U/L |
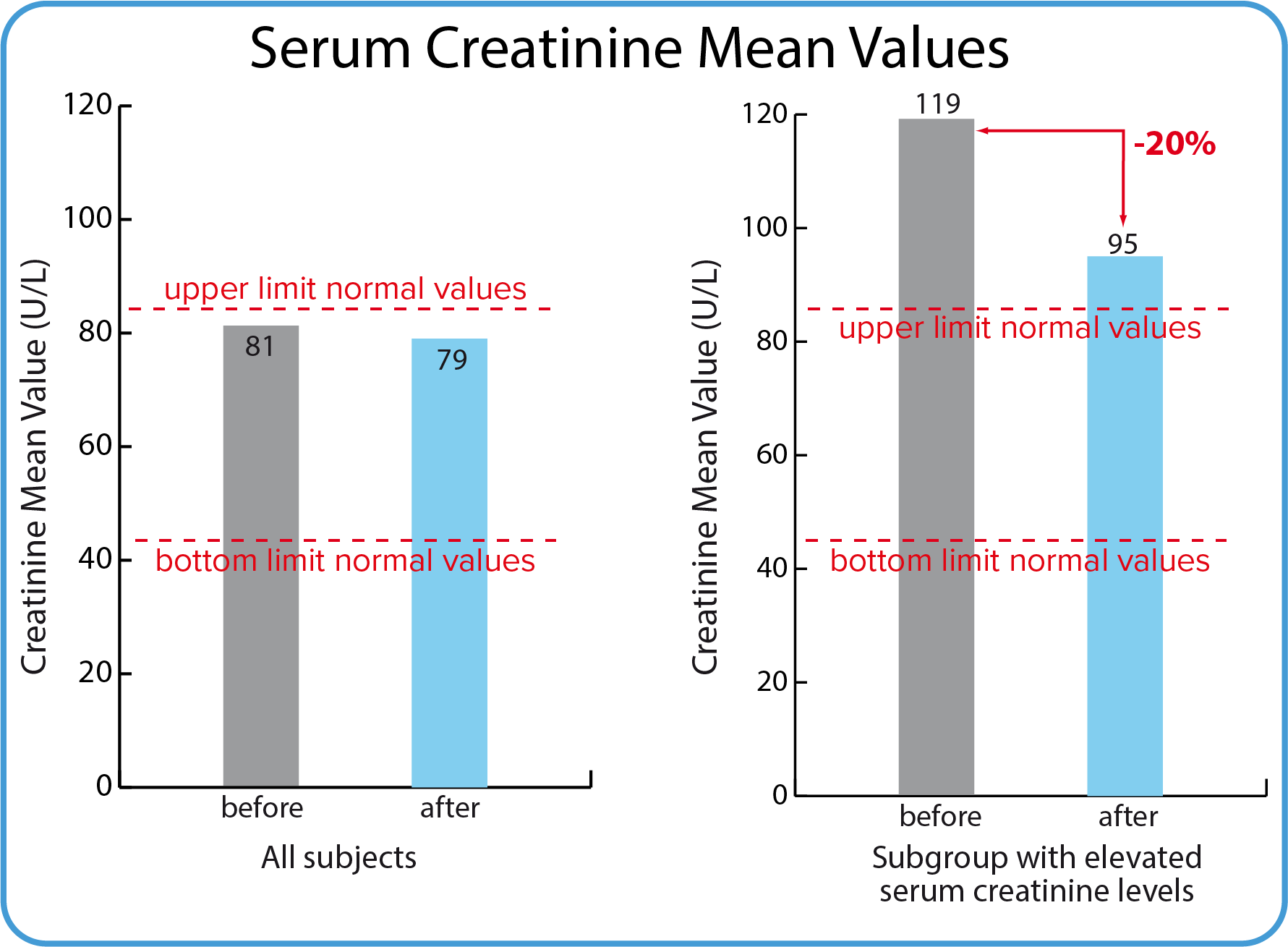
| Mean s-creatinine values for the whole group were normal before and after BodyDetox24 | Mean s-creatinine values for a group of participants with elevated creatinine values were reduced by 20 % after BodyDetox24 treatment. The significance of this is not known, but it might indicate an improved kidney function. |
Electrolytes
Potassium (K+), Sodium (Na+), Magnesium (Mg+2)
BodyDetox24 contains magnesium sulfate (so called bitter salt/Epsom salt) – the ingredient that empties the colon. The magnesium sulfate shows laxative effect. Xylitol, aloes, and plant extracts contained in the preparation may have in addition a certain laxative effect. A serum electrolyte test checks to see if this could have a negative effect. The action of magnesim sulfate is described in more detail on the tab presenting the composition of the BodyDetox24.
.
| Serum electrolyte | Before treatmment | After treatmment | Norm |
|---|---|---|---|
| Potassium (K+) | 4,4 mmol/L | 4,3 mmol/L | 3,6 - 5,0 mmol/L |
| Sodium (Na+) | 140 mmol/L | 139 mmol/L | 137 - 145 mmol/L |
| Magnesium (Mg+2) | 0,87 mmol/L | 0,86 mmol/L | 0,71 - 0,94 mmol/L |
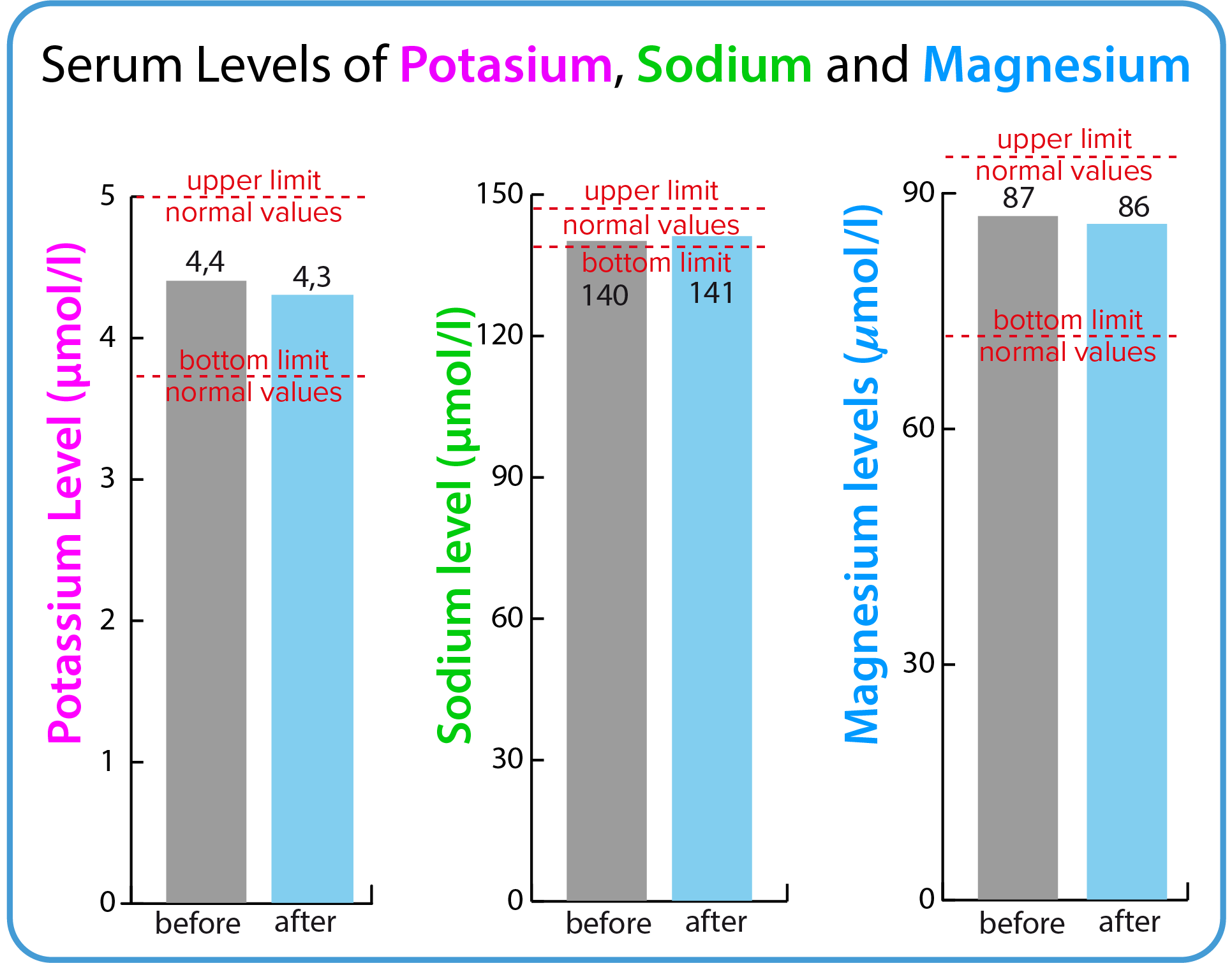
The Epsom salt is not absorbed by the body and it passes through the gut carrying water and waste products with it (osmotic laxative). No changes were seen in mean serum level of magnesium, mean potassium, and mean sodium values, indicating that using BodyDetox24 will not cause electrolytic disturbances. The results indicate that BodyDetox24 is safe to use.
Lactate dehydrogenase (LD)
Serum LD is a non-specific marker of cell damage. LD is a cytoplasmic enzyme that catalyzes the last step of glycolysis - the transition of pyruvate to lactate and vice versa. There are five types of LD that are known as isoenzymes. The five isoenzymes are found in different amounts in tissues throughout the body. LD-1: found in heart and red blood cells, LD-2: found in white blood cells. It is also found in heart and red blood cells, but in lesser amounts than LD-1, LD-3: found in lung tissue, LD-4: found in white blood cells, kidney and pancreas cells, and lymph nodes, LD-5: found in the liver and muscles of skeleton. The LD level increases in case of organs damage, e.g. heart attack, muscle damage, liver damage, pneumonia.
| Before treatment | Before treatment | Norm | |
|---|---|---|---|
| The whole group | 171 U/L | 159 U/L | 205 U/L |
| Participants with values above the reference before or after the test |
235 U/L | 182 U/L | 205 U/l |
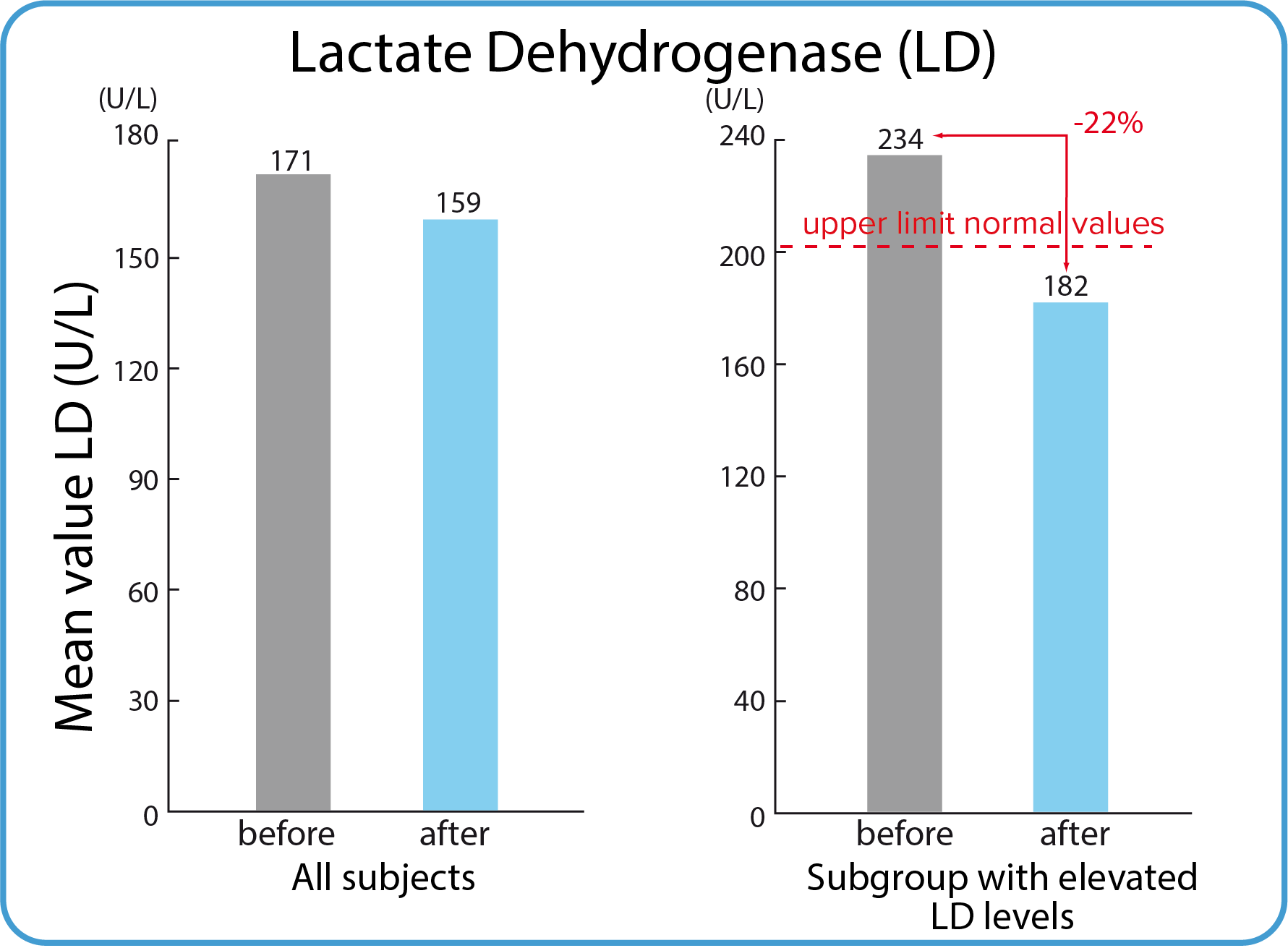
| Mean serum-LD values for the whole group were below the reference value and further reduced (< 205 U/L). | Five participants had s-LD valued above the reference before using BodyDetox24. The mean of their values was afterwards reduced by 22 % to normal. |
2. Results – Self-evaluation
A total of 56 volunteers participated and filled in a self-evaluation form, noting their personal experiences before and after using BodyDetox24 on a 5-step scale regarding changes – if any – in Energy, Tiredness, Sleep, and General well-being. The scale was numbered from 0 (No) to 4 (Very much) for the parameters Energy, Sleep, and Well-being. For Tiredness the 5-step scale was reversed.
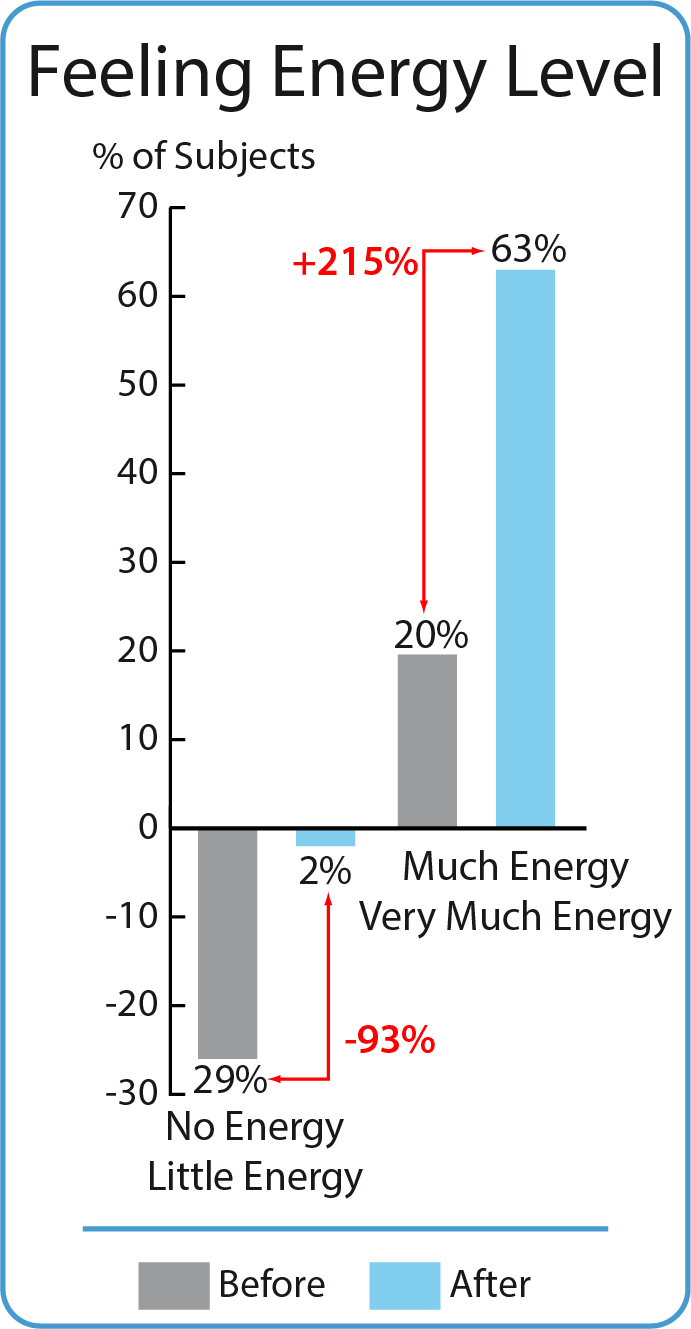
Assessment of the feeling of energy level before and after BodyDetox24 treatment
|
|
||
|
Click to magnify |
||
Sixteen participants (29 %) felt no energy or little energy before taking the BodyDetox24 treatment. This group has dropped to just one person (reduction by as much as 93%). The 20% of the study group, which initially felt energetic (much or very much energy) rose to 63% - an increase by 215% (increase more than 3-times).
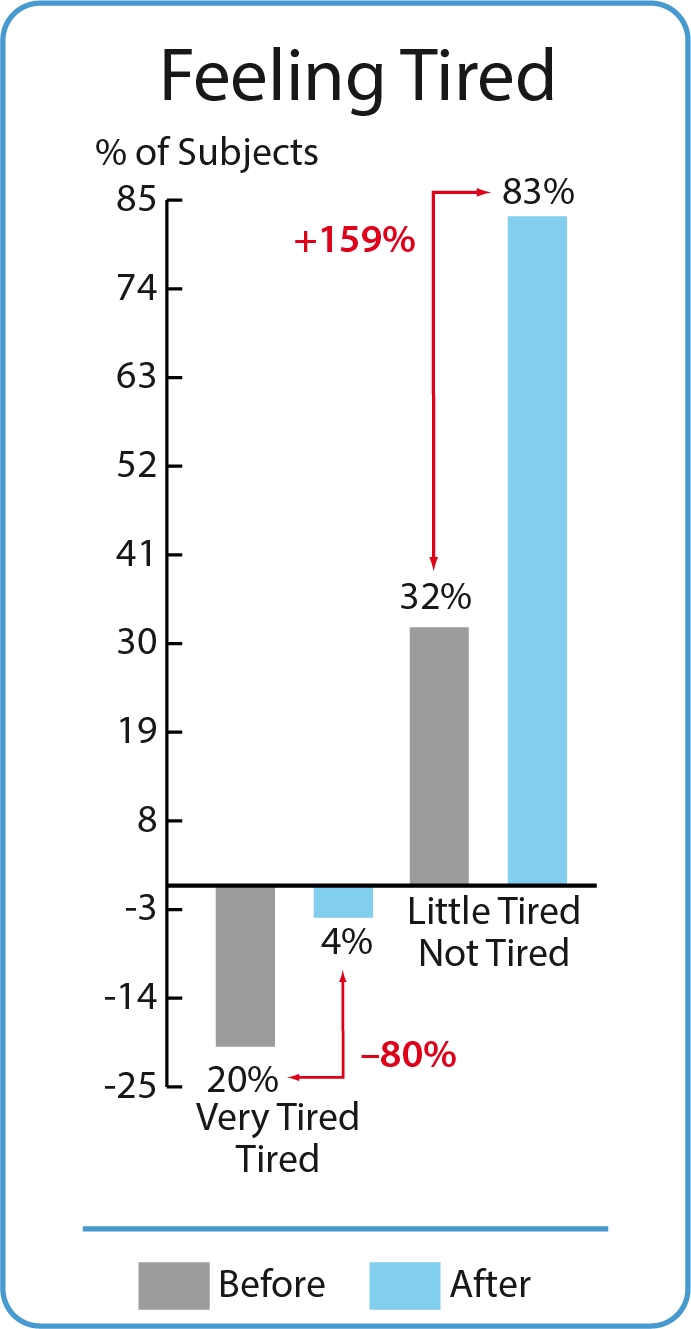
2. Evaluation of the tiredness feeling before and after BodyDetox24 treatment
|
|
 |
|
|
Click to magnify |
||
Eleven participants (20 %) felt very tired or tired before taking the BodyDetox24 treatment. This group has dropped to just two persons (reduction by 80 %). The group of study participants who felt slightly tired or not tired before the BodyDetox24 treatment increased from 32% to 83% after the treatment (an increase of more than two and a half times).
Eleven participants (20 %) felt very tired or tired before taking the BodyDetox24 treatment. This group has dropped to just two persons (reduction by 80 %) This group dropped to 2% - a reduction with 93% The 20% of the study group, which initially felt energetic (much or very much energy) rose to 63% - an increase by 215%. Przed kuracją BodyDetox24 20% badanych podało, że czuło się bardzo zmęczonymi lub zmęczonymi. Grupa ta zmniejszyła się o 80% po kuracji. Grupa uczestników badania, którzy przed kuracją BodyDetox24 czuli się mało zmęczonymi lub niezmęczonymi zwiększyła się po kuracji z 32% do 83% (wzrost ponad dwa i pół razy).
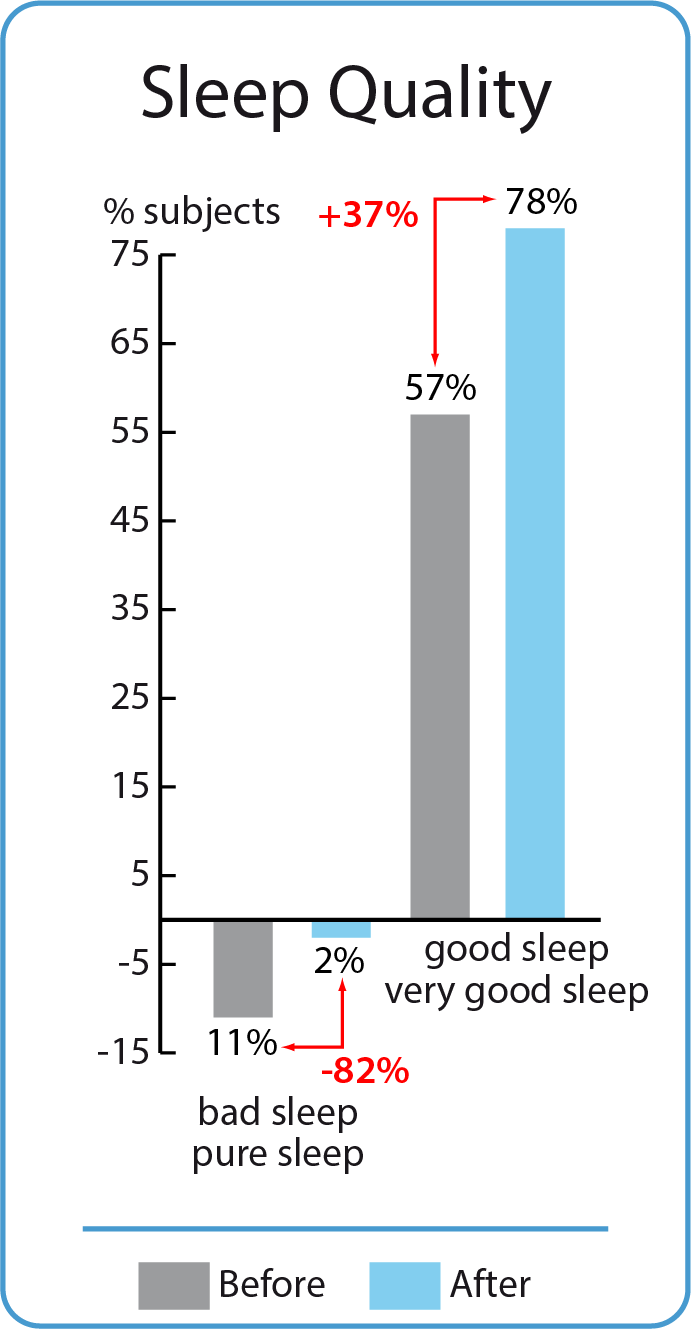
3. Assessment of sleep quality before and after BodyDetox24 treatment
|
|
 |
|
|
Click to magnify |
||
A minority (11%) the participants slept not well or just a little well before taking BodyDetox24. This group declined further after BodyDetox24. The group that slept well or very well before taking BodyDetox24 (57%) increased with 37% up to 78%
4. Assessment of geneal well-being before and after BodyDetox24 treatmment
The parameter “General well-being” was in the final analysis judged to be too unspecific and meaningless, and was therefore not taken into consideration for evaluation.
Discussion
This pilot study was performed on healthy volunteers. Mean values for biochemical blood parameters were therefore within normal (below or within reference values). Single liver markers (serum ALAT, serum GGT) outside normal values were improved but not normalized. This could be interpreted as using BodyDetox24 might have a positive effect on the liver.
Mean kidney function values (serum creatinine) were normal for the whole group before and after using BodyDetox24. For a small group of participants the mean value was slightly elevated before using BodyDetox24. This mean value was reduced by 20% but not normalized. The significance of reducing a mean serum creatinine value for a group of individuals is not known, but it might be interpreted as an improved kidney function in this sub-population.
No changes in serum electrolytes (potassium, sodium) or magnesium indicates that BodyDetox24 does not cause electrolyte disturbances. Specifically, the normal value for magnesium indicates that the magnesium salt used as an osmotic laxative (Epsom salt, magnesium sulphate) is not absorbed.
Mean values of the non-specific marker of cell damage, lactate dehydrogenase (serum LD), were also within normal range before and after BodyDetox24. Elevated mean values before using BodyDetox24 recorded for a minor group of participants were reduced by 22% to normal, which might be interpreted as an indication of a slight cell repair.
Basically, this small open-label pilot test confirms that BodyDetox24 is safe to use, as indicated by the toxicological data and previous experiences with BodyDetox24. The product functions without causing any harm as indicated by blood analyses. Furthermore, after using BodyDetox24 a majority of the volunteers felt more energetic, less tired and slept better.
Conclusions
- The BodyDetox24 cleansing treatment was well tolerated by all study participants, both in the subjective assessment and in the biochemical blood tests.
- The participants that showed elevated levels of liver enzymes and creatinine initially, there was a tendency for their normalization.
- The majority of the subjects experienced a clear subjective improvement in well-being after the BodyDetox treatment:
- Reducing the feeling of tired
- Reducing the feeling of lack/small energy
- Improving sleep quality.
- The changes listed above were observed very quickly, just in few days after the BodyDetox24 treatment.